© 2026 Biosurgica. Created with ❤ using WordPress and Kubio

Training & Capacity Building
Training: Educate staff on proper gowning, handling, and preventing cross-contamination.
DRAP regulatory training workshops
GMP / GDP / ISO training
Pharmacovigilance & Materiovigilance training
Medical Device Rules 2017 & Amendments training
Customized corporate regulatory training programs

Our team of experts not only work for in house but also deliver smart design solutions for cleanrooms and controlled airflow systems, engineered to meet the highest standards of contamination control and environmental precision to external clients. Our designs optimize airflow patterns, pressure differentials, and energy efficiency to support pharmaceutical, healthcare, and high-tech manufacturing environments. By combining engineering expertise with regulatory compliance, we create cleanroom solutions that ensure operational reliability, product protection, and long-term performance.

One of our company segments specialized in delivering reliable storage and cold chain management solutions designed to preserve product quality, safety, and compliance across every stage of the supply chain. Our temperature-controlled facilities and monitoring systems support pharmaceuticals, healthcare products, and sensitive materials, ensuring consistent conditions, full traceability, and regulatory alignment. With a focus on precision, reliability, and risk mitigation, we help our clients protect the integrity of their products from storage to distribution.

We provide advanced dehumidification solutions engineered for HVAC systems and critical pharmaceutical environments where precise, very low humidity (≤ 25%) control is essential. Our systems are designed to meet the stringent requirements of cleanrooms, manufacturing areas, storage facilities, and laboratories, ensuring product integrity, regulatory compliance, and process reliability. With a focus on performance, efficiency, and validation-ready solutions, we support controlled environments that demand uncompromising humidity management.
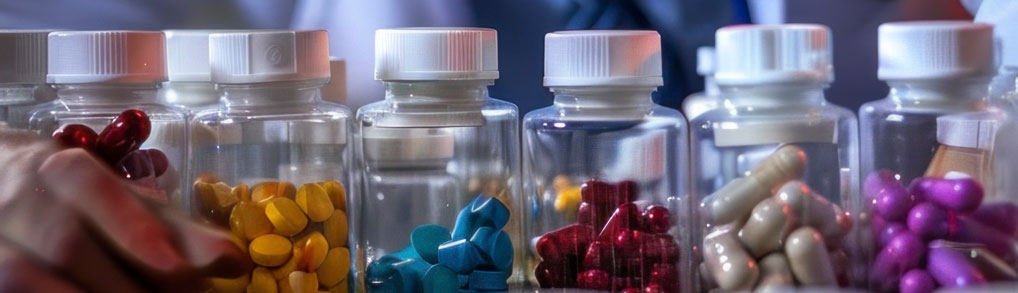
We offered complete solution for DRAP related documentation. From new company registration to existing Company documentation, dossier formation and much more.
A. Drugs / Pharmaceuticals
B. Biologicals
C. Medical Devices & IVDs
Regulatory hearing support
DRAP correspondence & follow-ups
Online DRAP portal submissions
Meeting representation with DRAP officials
Deficiency response preparation
B. Biologicals
C. Medical Devices & IVDs

Pharmaceutical HVAC Solutions
GMP-Compliant Environmental Control for Pharma Facilities
We deliver specialized HVAC solutions for the pharmaceutical industry, ensuring WHO-GMP, and DRAP compliance. Our systems protect product quality, ensure regulatory approval, and support uninterrupted manufacturing.
Cleanroom HVAC Systems
Temperature & Humidity Control
GMP HVAC Design & Consultancy
HVAC Validation & Testing
Automation & Monitoring
Energy-Efficient Solutions
Specialized Pharma Areas
Why Choose Us
Ready to Build a GMP-Compliant Facility?
From new pharmaceutical plants to HVAC upgrades, we deliver reliable, compliant, and efficient solutions.
Contact us today to discuss your pharmaceutical HVAC requirements.
A. Drugs / Pharmaceuticals
B. Biologicals
C. Medical Devices & IVDs